THIS WEBSITE USES COOKIES
We use cookies to personalise content, to provide social media features, and to analyse our traffic. By choosing 'allow all cookies', you consent to our cookies.
To find out more, read our privacy policy and cookie policy.
Diabetes concerns a large and rapidly growing patient group. Type 1 diabetes results from the autoimmune destruction of insulin-producing beta-cells distributed within the pancreas. There are currently two main treatments for diabetes: insulin therapy and transplantation.

The primary treatment for diabetes type 1 today involves exogenous insulin therapy, administrated trough lifelong daily injections or a pump, sometimes supplemented with device-based technologies to regulate blood glucose levels. However, constantly self-managing blood glucose levels poses not only a mental burden for persons with type 1 diabetes, but also makes it difficult to attain perfect blood sugar levels. Imperfect regulation may lead to the development of chronic complications, such as nephropathy, retinopathy, neuropathy, ischemic heart disease, peripheral vascular disease.

Pancreas transplantation effectively restores blood glucose control; however, the invasive procedure involved is linked to a high risk of complications. In contrast, intraportal islet transplantation offers a less invasive alternative, but it is less effective and poses challenges in maintaining long-term control. Both transplantation options are rarely preformed due to donor shortages and the requirement for lifelong antirejection medication.
Transplantation of the whole pancreas or isolated islets of Langerhans is an efficient way of treating type 1 diabetes. However, it is less frequently implemented due to its limitations such as donor shortage and lifelong immunosuppressive medication.
Developing a vascularized, personalized and immune-protected bioartificial pancreas capable of transplantation into non-immunosuppressed persons with type 1 diabetes addresses all the issues mentioned. Thus, the aim of the VANGUARD project was to generate a bioartificial pancreas for treating type 1 diabetes without the requirement for lifelong antirejection medication.
The development of a bioartificial pancreas was in the ‘preclinical developmental stage’ as per 2025 (see the infographic).
VANGUARD was a five-year research project primarily focused on advancing the generation of a bioartificial pancreas for persons with type 1 diabetes, progressing it through the preclinical development stage and moving it closer to market readiness as an Advanced Therapeutic Medicinal Product.
Furthermore, the project delved into the ethical, legal and psycho-social aspects related to the development of a bio-artificial pancreas. Researchers’ assed the perspectives of diabetes professionals and persons with type 1 diabetes regarding the bioartificial pancreas as a potential treatment option. This research contributed to ethically responsible clinical translation and implementation of this prospective therapy for type 1 diabetes.
Follow-up research resulting from the VANGUARD project will drive the advancement of the bioartificial pancreas into the Clinical Trials phase over the next fifteen years.
The timeline provided below illustrates an overview of clinical research stages involved in the development of the bioartificial pancreas. The timeline shows that the VANGUARD project aimed to successfully complete the Preclinical Development stage and produce a cell therapy in the form of an Advanced Therapy Medicinal Product.
Overall, the timeline from Preclinical Development to market launch typically spans between 10 to 15 years. However, developing an Advanced Therapy Medicinal Product like the bioartificial pancreas may require additional time due to associated legal and regulatory complexity of the procedure.
Click on the info signs on the infographics below to open up further information on stages of the Clinical Study Timeline.
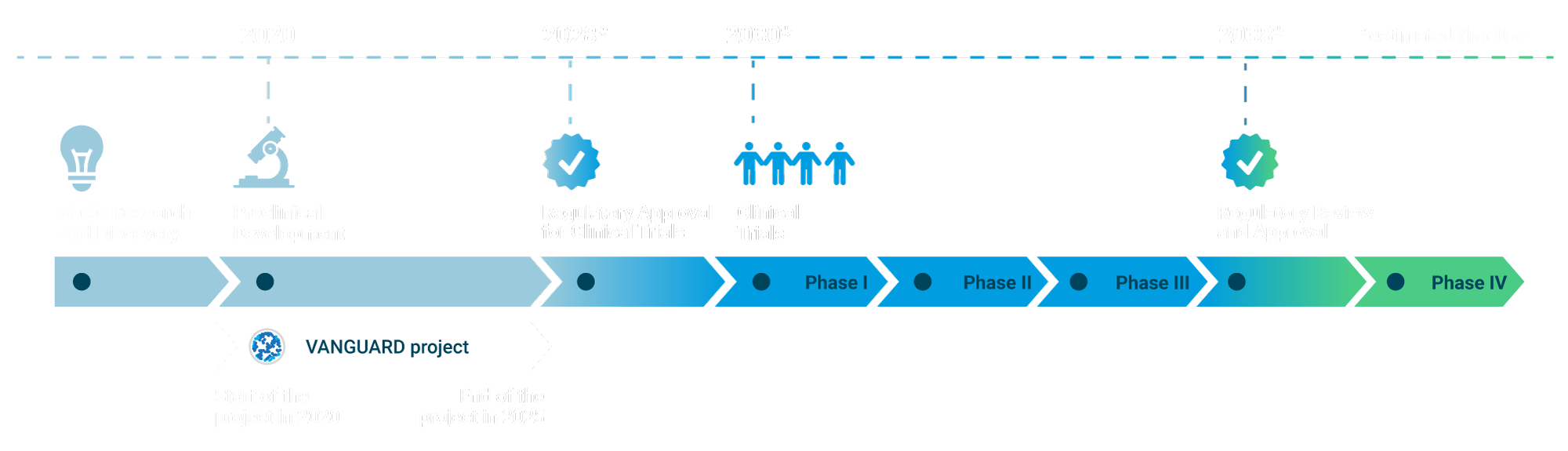
Basic Research and Discovery:
This initial phase involves understanding disease mechanisms and identifying potential therapeutic targets.
Preclinical Development:
This stage involves in vitro (lab-based) and in vivo (animal model) studies to assess the safety and efficacy of the therapy.
Regulatory Approval for Clinical Trials:
Before advancing to human trials, it is necessary to obtain regulatory approval from the European Medicines Agency. This process entails submitting an application, which typically takes 2-3 years under the best circumstances. Additionally, approval from an ethics committee is often necessary, which can further extend the timeline.
Phase I Clinical Trials:
These trials assess the safety of the therapy in a small group of individuals. The duration of this phase may span 1-2 years, depending on the outcomes.
Phase II Clinical Trials:
This phase evaluates the effectiveness of the therapy in a larger participant group and conducts additional safety assessments. This stage typically lasts 2-3 years.
Phase III Clinical Trials:
These trials are conducted on a larger scale to confirm the therapy's effectiveness and closely monitor any side effects. This phase typically spans 2-4 years.
Regulatory Review and Approval:
Following successful clinical trials, the therapy is submitted for regulatory approval. This review process typically lasts 1-2 years.
Post-Market Surveillance (Phase IV):
Following approval, the therapy undergoes ongoing monitoring for long-term effects in a larger population.
Basic Research and Discovery
This initial phase involves understanding disease mechanisms and identifying potential therapeutic targets.

Preclinical Development
This stage involves in vitro (lab-based) and in vivo (animal model) studies to assess the safety and efficacy of the therapy.

Regulatory Approval for Clinical Trials
Before advancing to human trials, it is necessary to obtain regulatory approval from the European Medicines Agency. This process entails submitting an application, which typically takes 2-3 years under the best circumstances. Additionally, approval from an ethics committee is often necessary, which can further extend the timeline.

Phase I Clinical Trials
These trials assess the safety of the therapy in a small group of individuals. The duration of this phase may span 1-2 years, depending on the outcomes.
Phase II Clinical Trials
This phase evaluates the effectiveness of the therapy in a larger participant group and conducts additional safety assessments. This stage typically lasts 2-3 years.
Phase III Clinical Trials
These trials are conducted on a larger scale to confirm the therapy's effectiveness and closely monitor any side effects. This phase typically spans 2-4 years.

Regulatory Review and Approval
Following successful clinical trials, the therapy is submitted for regulatory approval. This review process typically lasts 1-2 years.
Post-Market Surveillance (Phase IV)
Following approval, the therapy undergoes ongoing monitoring for long-term effects in a larger population.
To learn more about the Preclinical Development stage of bioartificial pancreas research led by VANGUARD, please consult the About the Project page.
In this section, you will find answers to some of the most common questions about the VANGUARD project.
VANGUARD was an innovative research project aiming to generate a vascularized, personalized and immune-protected bioartificial pancreas by combining advanced tissue engineering strategies to treat type 1 diabetes. Its consortium consisted of 6 research institutions, 2 SMEs and 1 NGO with expertise in science, clinics, ethics and industry. This project has received funding from the European Union’s Horizon 2020 research and innovation programme and ended in June 2025.
The VANGUARD project was a five-year research initiative that began in 2020. It was anticipated to progress through the Preclinical Development stage of bioartificial pancreas development.
The VANGUARD project consortium comprised partners from five countries: Switzerland, Italy, France, Germany, and the Netherlands. Each partner played a specific role in the project, with activities conducted within their respective locales. Additionally, the consortium conducted collaborative activities through both online and in-person meetings.
Bioartificial pancreas is a form of an Advanced Therapy Medicinal Product for cell therapy of type 1 diabetes. A vascularized and immune-protected bioartificial pancreas can be transplanted into non-immunosuppressed patients; it combines advanced tissue engineering strategies, such as 3D organoid generation, hydrogel design, bioartificial organ assembly and CRISPR-Cas9 gene editing.
The development of a bioartificial pancreas was in the Preclinical Development stage in 2025 when the VANGUARD project was completed. The VANGUARD team foresees that the research will progress towards achieving market readiness for the bioartificial pancreas as an Advanced Therapy Medicinal Product to treat type 1 diabetes by around 2038.