THIS WEBSITE USES COOKIES
We use cookies to personalise content, to provide social media features, and to analyse our traffic. By choosing 'allow all cookies', you consent to our cookies.
To find out more, read our privacy policy and cookie policy.
9 partners from 5 countries
€ 6.8 million
01.01.2020 – 30.06.2025
Type 1 diabetes results from the autoimmune destruction of insulin-producing beta-cells. These cells are located in mini-organs, called islets of Langerhans, which are distributed within the pancreas. The disease usually starts in childhood or young adulthood. It is the most common chronic disease in children and adolescents. Type 1 diabetes can be cured by transplantation, either of the whole pancreas or of isolated islets. Europe is the region with the highest reported number of children and adolescents affected by Type 1 diabetes.
Pancreas transplantation is an efficient procedure for restoring blood sugar control. However, this is a major surgical procedure, plagued by a high complication rate. It is only performed in a minority of patients with type 1 diabetes because of the marked imbalance between organ donors and individuals suffering from the disease.
Cell therapy for type 1 diabetes is currently performed in a small number of selected patients by transplantation of allogeneic islets of Langerhans, with good functional outcomes. Islet transplantation is a valuable first step toward offering a cure for all patients with type 1 diabetes but this therapeutic approach is hampered by several issues:
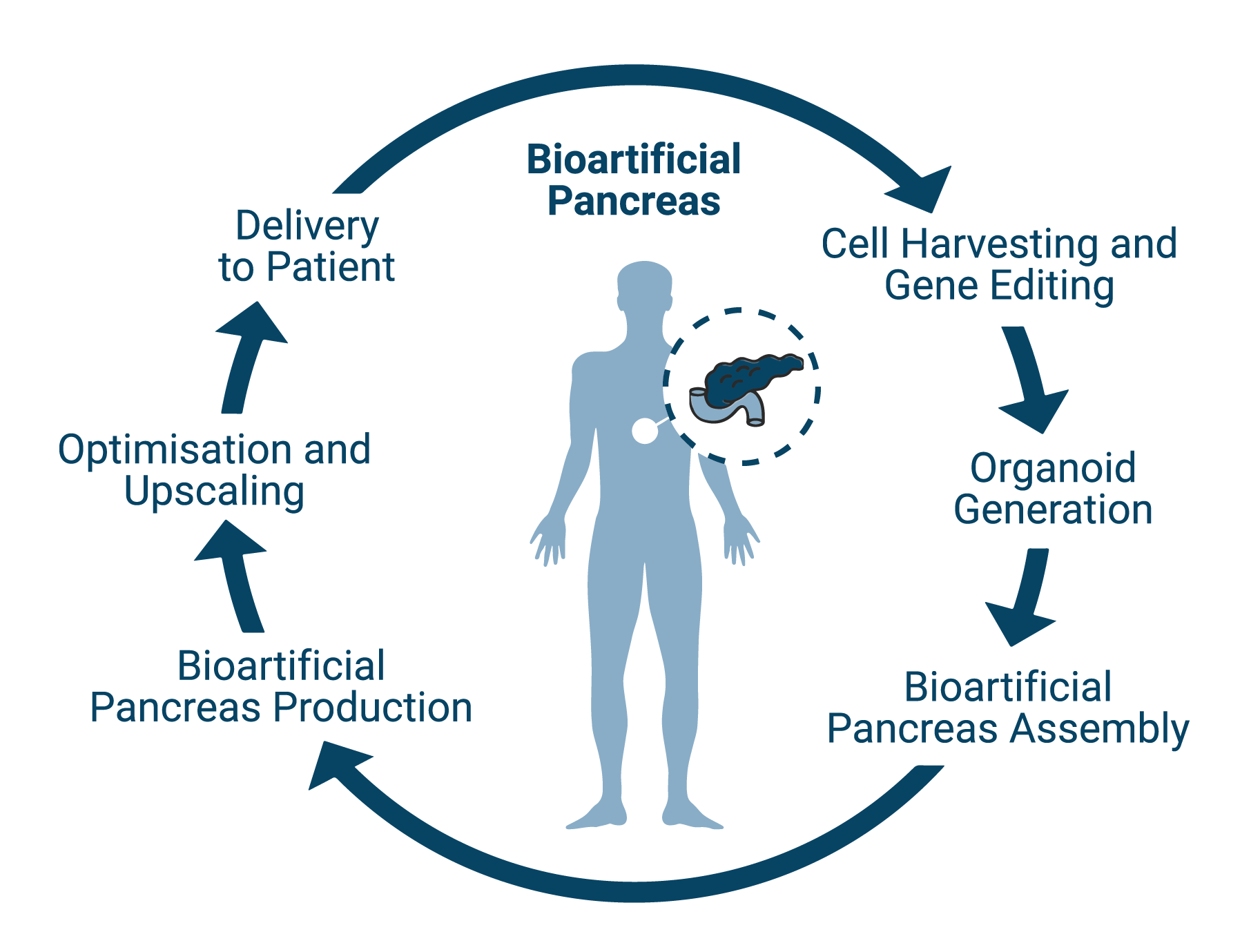
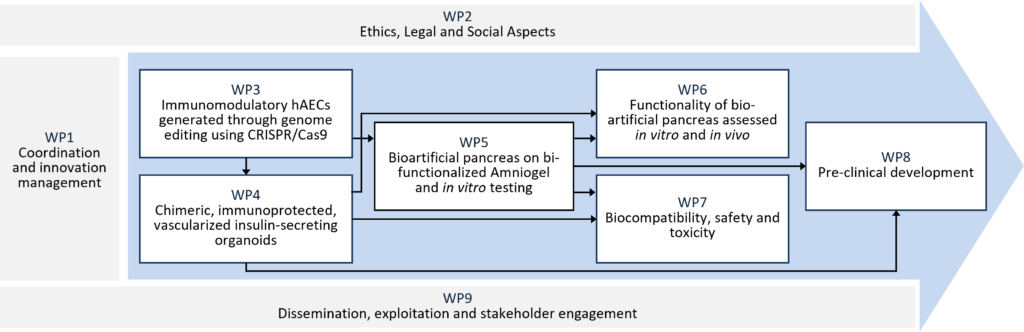
The VANGUARD project aimed to generate a vascularized and immune-protected bioartificial pancreas that can be transplanted into non-immunosuppressed patients; it combined advanced tissue engineering strategies, such as 3D organoid generation, hydrogel design, bioartificial organ assembly and CRISPR-Cas9 gene editing. The bioengineering of insulin-producing 3D organoids and their assembly into a bioartificial endocrine pancreas was based on these components:
Organoid manufacturing was performed using the Sphericalplate 5D, a patented cell culturing platform to be medically approved as a medical device class IIa.

This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 874700